Abstract
Background Crovalimab is a novel anti-human C5 monoclonal antibody being developed for the treatment of PNH, a rare, life-threatening, acquired hematopoietic stem cell disorder. Crovalimab is a next-generation C5 inhibitor that enables rapid and sustained C5 inhibition with subcutaneous (SC) self-administration every 4 weeks.
COMPOSER (NCT03157635) is a 4-part open-label adaptive Phase I/II trial evaluating crovalimab in healthy volunteers and in patients with PNH who were treatment-naive or switched from eculizumab to crovalimab. COMMODORE 3 (NCT04654468) is a multicenter, single-arm, Phase III study evaluating crovalimab in treatment-naive patients with PNH in China. Crovalimab dosing and regimen were optimized in COMPOSER (Sostelly ASH 2019) and are currently being investigated in pivotal studies, including COMMODORE 3. The dose and regimen were:
Week 1, Day 1: 1000 mg IV for body weight 40 to <100 kg or 1500 mg IV for weight ≥100 kg
Week 1, Day 2 and Weeks 2, 3, and 4: 340 mg SC
Week 5 onward: 680 mg SC for weight 40 to <100 kg or 1020 mg SC for weight ≥100 kg, Q4W
The objectives were to characterize crovalimab's pharmacokinetics (PK) in healthy volunteers and treatment-naive patients with PNH, and to characterize the relationship between crovalimab's exposure parameters and pharmacodynamic (PD) biomarkers, efficacy, and safety, using data from COMPOSER and COMMODORE 3.
Methods The legacy population PK model (Sostelly ASH 2019) was updated using data from healthy volunteers and treatment-naive patients from COMPOSER and COMMODORE 3 to characterize the crovalimab PK profile and evaluate impact of potential covariates.
Simulations were conducted using the population PK model to evaluate the need for dose adjustment in the population based on the identified significant covariates.
The relationships between crovalimab exposure and PD biomarkers, efficacy (normalized lactate dehydrogenase [LDH]), and safety endpoints were graphically explored. PD biomarkers included markers of terminal complement activity (50% hemolytic activity [CH50] as measured by liposome immunoassay [LIA]) and free C5. Safety endpoints included adverse events (AEs) of special interest, related serious AEs, related Grade ≥3 AEs, and any infections.
Results Crovalimab PK was best described by a linear two-compartment model with first-order elimination and absorption. After SC administration, bioavailability was estimated to be 73.8% with a typical terminal half-life of 58.7 days (90% CI: 50.7, 74.1). Crovalimab clearances and volumes of distribution were allometrically scaled by body weight; patients with lower weight may experience relatively higher drug exposure vs those with higher weight. Age was found to be negatively correlated with the absorption rate constant without a clinically relevant impact on crovalimab exposure.
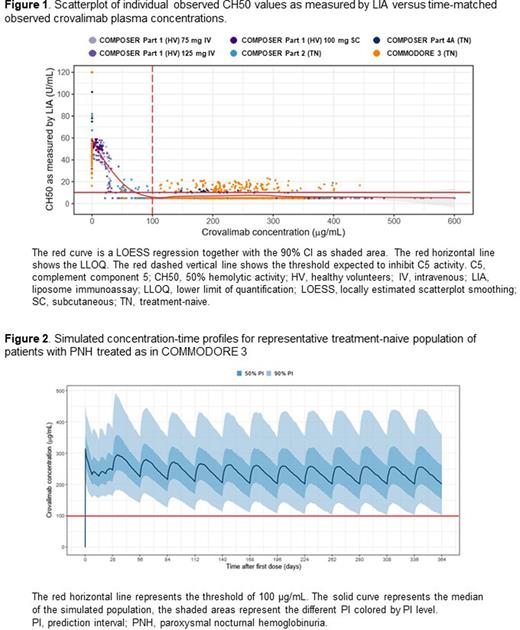
The PK/PD relationship showed that crovalimab induced a concentration-dependent inhibition of terminal complement activity (Figure 1) and free C5 concentrations. Crovalimab concentrations above approximately 100 μg/mL resulted in inhibition of terminal complement activity as indicated by low CH50 values (LIA close or below the lower limit of quantification [10 U/mL]) and low free C5 (<0.0001 g/L in COMMODORE 3).
No relationship was identified between observed steady-state crovalimab concentrations and LDH. For concentrations above approximately 100 μg/mL, no further LDH reduction could be achieved with higher concentrations. The recommended dosing regimen delivered adequate crovalimab concentration to achieve the desired LDH reduction.
No relationship between crovalimab exposure and AE occurrence was identified, in line with other C5 inhibitors. Using the recommended dose and regimen, simulations of a representative treatment-naive patient population (based on patient characteristics across studies) showed that the predicted trough concentration at steady state remained above the threshold of 100 µg/mL in >95% of simulated patients (Figure 2).
No dose adjustment was required for other intrinsic factors such as ethnicity, anti-drug antibody status, and hepatic or renal impairment.
Conclusion PK characterization and exposure-response analyses supported the effectiveness and safety of the weight-based two-tiered crovalimab dosing approach for the treatment of adult and adolescent patients with PNH not currently treated with complement inhibitors.
Disclosures
Buatois:F. Hoffmann-La Roche Ltd.: Current Employment, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Benkali:Certara, Inc: Current Employment; F. Hoffmann-La Roche Ltd: Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Henrich:Pharmetheus: Current Employment; F. Hoffmann-La Roche Ltd: Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Jaminion:F. Hoffmann-La Roche Ltd.: Current Employment, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Zhang:China Innovation Center of Roche: Current Employment; F. Hoffmann-La Roche Ltd.: Other: All authors received support for third-party writing assistance, furnished by Bena Lim, PhD, of MediTech Media and Scott Battle, PhD, of Health Interactions and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Sostelly:F. Hoffmann-La Roche Ltd.: Current Employment, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal